0.9 Sodium Chloride Injection Usp Used For
Sodium Chloride Injection USP is indicated as a source of water and electrolytes. Download SODIUM CHLORIDE INJECTION USP 09 Product Sheet.

Bacteriostatic Sodium Chloride 0 9 For Injection 20ml Single Vial Mountainside Healthcare Com
Not manufactured with latex PVC or DEHP.

0.9 sodium chloride injection usp used for
. Sodium Chloride Injection USP. 09 Sodium Chloride Injection USP is indicated for extracellular fluid replacement treatment of metabolic alkalosis in the presence of fluid loss and mild sodium depletion. They are parenteral solutions containing various concentrations of sodium chloride in water for injection intended for intravenous administration. For intravenous use only.Announces FDA Approval of Halobetasol Propionate Ointment 005. No preservative antimicrobial agent or buffer is added. The small volume of fluid and amount of sodium chloride provided by 09 Sodium Chloride Injection USP when used only as an isotonic vehicle for parenteral injection of drugs is unlikely to exert a significant effect on fluid and electrolyte balance except possibly in neonates and very small infants. 09 Sodium Chloride Injection USP is indicated for extracellular fluid replacement treatment of metabolic alkalosis in the presence of fluid loss and mild sodium depletion.
Sodium Chloride Injection USP 09 is a formulation of sodium chloride in Water for Intravascular Injection. This product belongs to the Electrolyte Solutions group and is distributed under medical prescription. 09 Sodium Chloride Injection USP can be used as a vehicle or diluent for compatible products for parenteral administration. For 09 Sodium Chloride Injection USP each 100 mL contains 900 mg sodium chloride in water for injection.
Procedures and may be used to initiate and terminate blood transfusions without hemolyzing red blood. 09 Sodium Chloride Injection USP is indicated for extracellular fluid replacement treatment of metabolic alkalosis in the presence of fluid loss and mild sodium depletion. It is a parenteral solution containing sodium chloride in water for injection intended for intravenous administration. 09 Sodium Chloride Irrigation USP is utilized for a variety of clinical indications such as sterile irrigation of body cavities tissues or wounds indwelling urethral catheters surgical drainage tubes and for washing rinsing or soaking surgical dressings instruments and laboratory specimens.
Between 45 and 70. 09 Sodium Chloride Injection USP is also indicated for use as a priming solution in hemodialysis procedures. 09 Sodium Chloride Injection USP is also indicated for use as a priming solution in hemodialysis procedures. Dosage and Administration Warm to body temperature prior to administration.
09 Sodium Chloride Injection USP solutions are sterile and nonpyrogenic. Sodium Chloride 09 is a solution for injection and is used as a solvent and carrier for drugs. Sodium Chloride Injection USP 09 Indications. Sodium Chloride Injection USP should be used with great care if at all in patients with congestive heart failure severe renal insufficiency and in clinical states in which there exists edema with sodium retention.
Change lost body fluids and salts dilute other medications which might be provided by injection or drip act as a sterile liquid for cleaning wounds nasal passages or throughout surgery. Bacterial endotoxins 85 It contains not more than 05 USP Endotoxin Unit per mL where the labeled amount of sodium chloride in the Injection is between 05 and 09 and not more than 36 USP Endotoxin Units per mL where the labeled amount of sodium chloride in the Injection is between 30 and 243. Sodium Chloride Injection USP is also indicated as a pharmaceutic aid and diluent for the infusion of compatible drug additives. 09 Sodium Chloride Injection USP is sterile and nonpyrogenic.
It can be given by the intravenous intramuscular or subcutaneous route. An isotonic electrolyte solution as an aid in the treatment of dehydration and electrolyte disturbances associated with sodium and chloride in cattle swine horses sheep dogs and cats. 09 Sodium Chloride Inj. Sodium Chloride 09 Injection is used to.
09 Sodium Chloride Injection USP is also indicated for use as a priming solution in hemodialysis procedures.

Bacteriostatic Sodium Chloride 0 9 Injection 30ml Single Vial Mountainside Medical Equipment

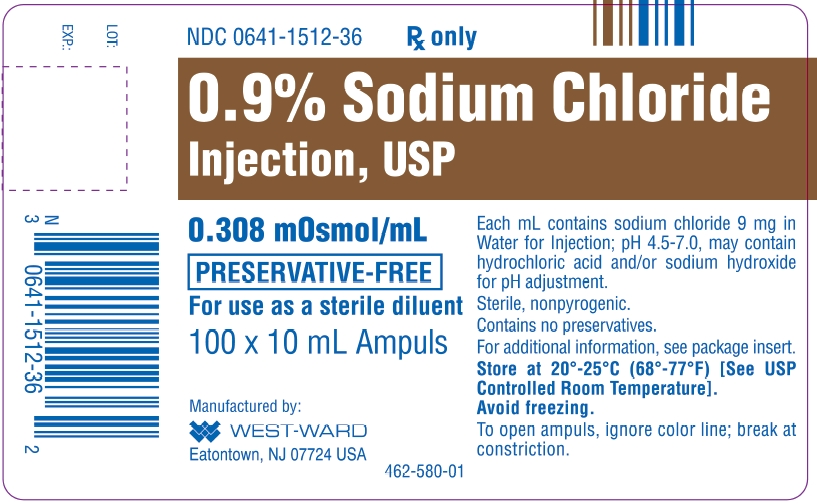
Sodium Chloride Injection Usp 0 9 Single Dose Ampules
Diluent Bacteriostatic 0 9 Sodium Chloride Injection By Pfizer Medline Industries Inc

Sodium Chloride Injection Usp 0 9 Single Dose Ampules

Bacteriostatic Sodium Chloride 0 9 For Injection 20ml Single Vial Mountainside Healthcare Com

Bacteriostatic 0 9 Sodium Chloride Injection 30ml Online

0 9 Sodium Chloride Injection 50ml 96 Cs Wolf Medical Supply
Post a Comment for "0.9 Sodium Chloride Injection Usp Used For"